No content results match your keyword.
Content
You have successfully logged out.
Not registered yet?
No content results match your keyword.
Content
No product results match your keyword.
Products
Stability starts on the surface
Introducing our advanced interbody fusion devices with a porous titanium coating and PEEK core. Experience the fusion of osteoconductive performance and bone-mimicking features. This is the choice of eXPerts.
Clinical performance
We commit ourselves to creating a solution that enhances surgical and biomechanical performance at every level. The result is PLASMAPOREXP®: a surface-enhancing coating that truly creates value for the best possible patient outcomes.
Osteoconductive texture provides
0%
greater performance in apposition* [1,2,4]
Finely balanced coating structure ensures
0%
more delamination resistance** [3]
Strong and elastic at the same time - the XP coated cages are
0%
closer to the elastic modulud of cortical bone.*** [2,5,6]
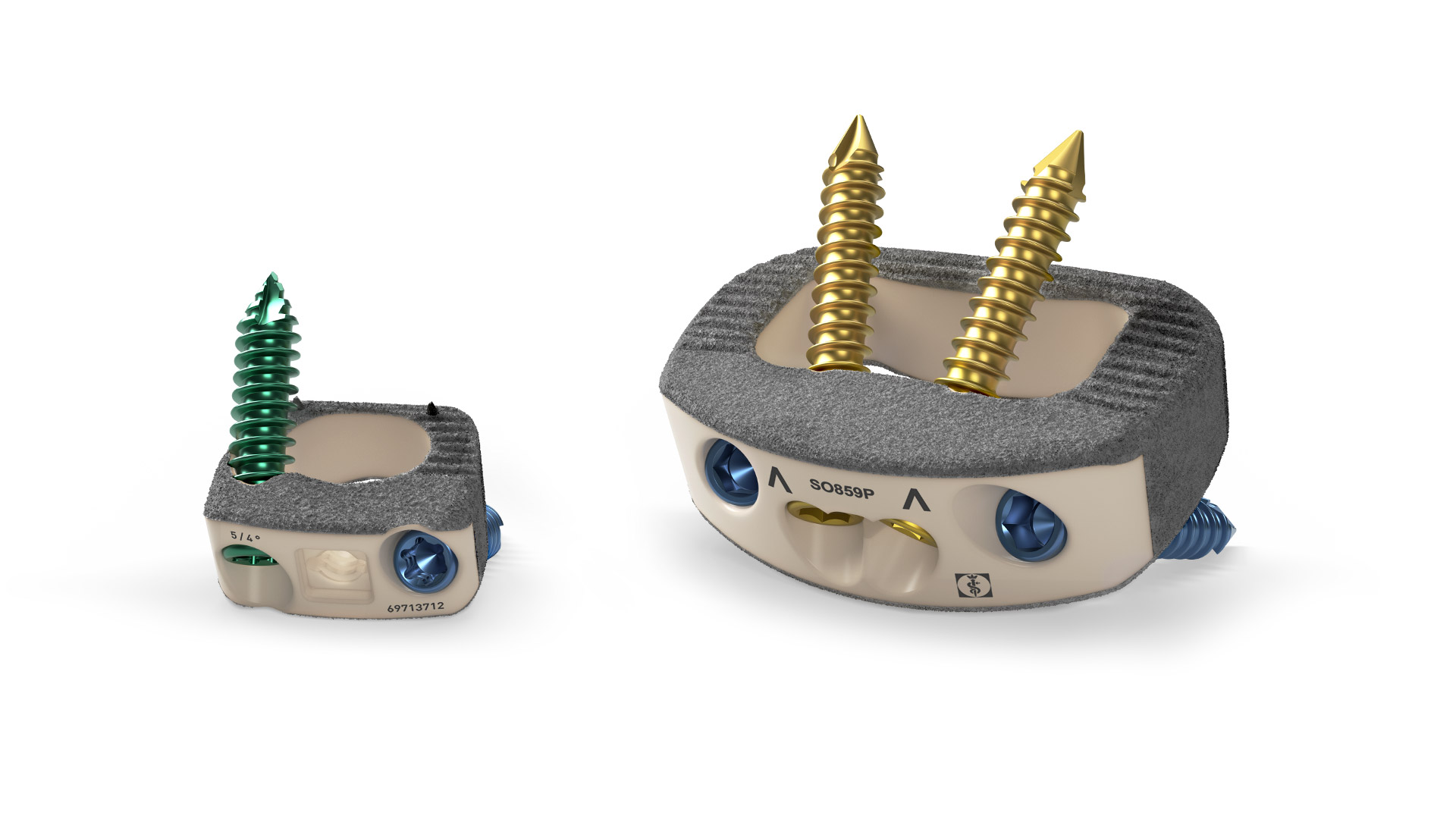
PLASMAPORE XP® enables clear visualization of implant contours, while the radiolucent nature of PEEK allows clear visibility on X-rays and CT scans, giving surgeons the images they need to assess the fusion process.
/
The porous titanium surface creates a very good surface-to-bone contact for enhanced osseointegration.[1,4]
/
All implants can be filled with bone or bone substitute for optimized fusion results.
/
Apposition
The design of the coating is well balanced and falls within the ideal range of porosity and pore size for bone ingrowth. This reduces the fibrous response while creating an osteoconductive environment that leads to early and long-term stability. [4]


Stability
Enhanced surface structure for high initial stability and longterm migration resistance. The cavity of the porous coating extends the surface area, providing great anatomical in terms of osteointegration and thus biomechanical advantages like pull-out strength. [1-4]
Elasticity
The combination of a PEEK core and our PLASMAPOREXP® creates a modulus of elasticity that comes very close to bone. This prevents the risk of subsidence and reduction in bone density. [2,5,6]


Visualization
The radiolucent core allows for enhanced intra- and post-operative imaging. The thickness of our PLASMAPOREXP® coating provides implant contours during imaging with low artifact formation in CT and MRI scans. Next to that, markers provide orientation for in-situ implant placement.
The portfolio
AESCULAP® Spine Surgery
* compared to uncoated interbody fusion devices, after 24 weeks of implantation
** compared to industry recommended requirement
*** compared to solid titanium alloy interbody fusion devices.
Your feedback matters! Participate in our customer survey to help us enhance our website, products and services. Thank you for your support!