Influence of surgery
Surgery is used to prevent, diagnose, stage and treat cancer. The approach for surgery can be curative or palliative. The purpose of surgeries often are strategies to debulk cancer or support cancer treatment, like port catheters or reconstructive surgery after major surgery. Sometimes, one surgery can accomplish more than one of these goals.1
Malnutrition and underfeeding are risk factors for postoperative complications.2,3 There is evidence that malnutrition is associated with a worse outcome. Additionally, there is enough evidence that major surgical stress and trauma will induce catabolism, which contributes to malnutrition.2
Malnourished cancer patients undergoing surgery showed a higher incidence of incision infection as well as lower 3-year overall and disease-free survival rates.4
Therefore, the ESPEN guideline for surgery recommends the Enhanced Recovery After Surgery (ERAS) concept and emphasizes the nutritional needs of patients undergoing major surgery, e.g., for cancer.2
The key aspects of perioperative care according to ESPEN include2:
- integration of nutrition into the overall management of the patient
- avoidance of long periods of preoperative fasting
- re-establishment of oral feeding as early as possible after surgery
- start of nutritional therapy early, as soon as a nutritional risk becomes apparent
- metabolic control, e.g., of blood glucose
- reduction of factors which exacerbate stress-related catabolism or impair gastrointestinal function
- reduced time on paralytic agents for ventilator management in the postoperative period
- early mobilization to facilitate protein synthesis and muscle function
Malnutrition and underfeeding are risk factors for postoperative complications and are associated with lower survival.
Influence of chemotherapy
If chemotherapy is used to treat cancer, it is important to understand the goals of treatment. The three main goals of chemotherapy in cancer treatment are cure, control or palliation.5
Chemotherapy drugs target cells at different phases of the cell cycle. More than 100 chemotherapy drugs are used to treat cancer—either alone or in combination with other drugs or treatments. Classical chemotherapy cannot differentiate between healthy and cancerous cells. This means that classical chemotherapy damages normal (healthy) cells as well as cancer cells, and this is the main reason for the adverse reactions.
Typical adverse reactions of chemotherapy that affect nutritional status6:
- Fatigue
- Mood changes
- Nausea and vomiting
- Appetite changes
- Constipation
- Diarrhea
- Weight changes
- Infection
- Mouth, tongue, and throat problems such as sores and pain on swallowing
Evidence
Regarding the mentioned adverse reactions, it seems clear that nutritional status may be affected by chemotherapy and this is shown in several studies.8-12
In a prospective study of 11 centers and 313 patients with gastrointestinal malignancies under chemotherapy, the prevalence of moderate and severe malnutrition was present in 27% and 25% of cases.8
Similar data was shown in a prospective study of 191 mixed cancer patients receiving chemotherapy. Around 58% of patients experienced some degree of weight loss. The prevalence was higher among gastro intestinal (excluding colorectal cancer) and lung cancer patients.11
Typical symptoms reported during chemotherapy were11:
- nausea (59.6%)
- anorexia (46%)
- constipation (31.9%)
Patients with ≥ 5% weight loss during chemotherapy more frequently showed anorexia, nausea and vomiting.11
In another descriptive cross-sectional study, the majority of patients reported that chemotherapy-induced nausea and vomiting (CINV) limited their nutritional intake.9 It is also well known that chemotherapy can lead to dysgeusia and therefore have a negative impact on food enjoyment.12 These results indicate that GI symptoms during chemotherapy promote weight loss in cancer patients.
Advanced cancer patients with chemotherapy and malnutrition receive fewer cycles of treatment13 and show increased risk of mortality.13,14
Most chemotherapy drugs are administered based on body surface area, which is a calculation that only accounts for height and weight. Pharmacokinetics (drug metabolism) occurs in muscle mass and hence lean tissue compartments.15
Based on this concept, a sarcopenic person would receive a large amount of drugs for a small lean tissue compartment, increasing this person’s risk of developing dose-limiting toxicity (DLT). DLT is an unfavorable and undesirable outcome of chemotherapy that leads to treatment termination, discontinuation, hospitalization or death.15 It was shown that sarcopenia increases the risk of dose-limiting toxicity.16,17 In a study with neoadjuvant chemotherapy, sarcopenia was more prevalent after treatment than before.18
During chemotherapy, GI symptoms are common and promote malnutrition in cancer patients, which is associated with a worse outcome.
Influence of radiation
Radiation therapy (RT) is the use of radiation to treat cancer by killing cancer cells. Special equipment sends high doses of radiation to the cancer cells or tumor to keep the cells from growing (making more cancer cells). Radiation can also affect normal cells near the tumor, but they are able to repair themselves while cancer cells cannot.19
The most common adverse reactions of radiation are19:
- eating problems (loss of appetite, dysphagia, mucositis)
- fatigue
- skin changes
Evidence
The German-Swiss-Austrian Group on Maxillofacial Tumors (DOESAK) rehab study of 1652 oral cancer patients showed that radiotherapy had a negative impact on diet and weight. Radiotherapy was the reason that patients much more frequently ate mashed or liquid food. Radiotherapy had a negative impact on mouth moisture, sense of taste and swelling. Patients who underwent radiotherapy suffered more from weight loss compared with other forms of treatment.20
In another study of patients undergoing radiation therapy, malnutrition was present in 31 % of all patients at the onset and increased to 43 % at the end of RT.21 This shows the importance of nutrition intervention strategies during treatment. Weight loss during radiation is associated with an impaired quality of life20 and significantly worse disease-specific survival.22
Two adverse reactions of radiation therapy need special focus regarding nutrition:
Radiation enteritis
Radiation enteritis is defined as the loss of the intestine's absorptive capacity following irradiation, which is most commonly seen after radiotherapy for pelvic and abdominal malignancies. These patients have a high risk of malnutrition due to diarrhea and malabsorption.23
Mucositis
The term “mucositis” was introduced in late 1980 to describe inflammation of the oral mucosa induced by radiotherapy (observed in 80 % of patients), chemotherapy (in 40 %to 80 % of patients) and bone marrow transplantation (in over 75 % of patients)—the phenomenon being regarded as a manifestation of leukopenia.24 Mucositis is a frequent complication and threatens the effectiveness of therapy because it leads to dose reductions, increases healthcare costs, and impairs patients' quality of life.25 The consequences of oral mucositis include infection, xerostomia, hemorrhage and nutritional deficits.26 Malnutrition is probably a risk factor for mucositis.27
Concurrent chemoradiotherapy (CCRT), e.g., for head and neck cancer leads to more fatigue, drowsiness, lack of appetite, problems with mouth / throat mucus and problems in tasting food than for those receiving RT alone.28
The incidence of oral mucositis in head and neck patients receiving CCRT is high—as an example, a recent study showed an incidence of 33.3% at the end of the 1st week, which increased up to 93.3% by the end of the 5th week and then had a decreasing trend within the 6th week (70.0%).29 Malnutrition in rectal cancer patients receiving preoperative CCRT is common (51%) and associated with lower treatment tolerability and anastomotic leakage.30
Malnutrition occurs often during RT, mainly in head and neck patients. CCRT shows more adverse reactions and patients have a high risk of malnutrition.
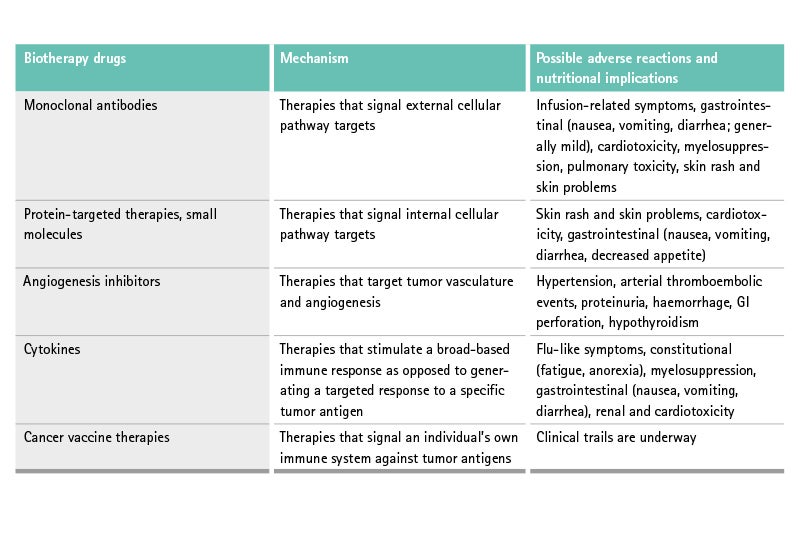
Influence of biotherapy
Biotherapy is defined as treatment to boost or restore the ability of the immune system to fight against the cancer. Biotherapy drugs are used alone or in combination with chemotherapy drugs. Some types of immunotherapy are also sometimes called biological therapy or biotherapy.
The functions of immunotherapy according to the National Cancer Institute are as follows31:
- to stop, control or suppress processes that allow cancer growth
- to make malignant cells more recognizable and thus more susceptible to destruction by the immune system
- to boost the killing ability of the immune system’s cells
- to prevent malignant cells from metastasizing to other locations in the body
- to eliminate malignant cells that have not been killed by other modalities of cancer treatment such as chemotherapy or radiotherapy
These kinds of therapies lead to different adverse reactions than “classical” chemotherapy due to vascular, dermatologic, endocrine, coagulation, immunologic, ocular, and pulmonary toxicities.32
The nutritional effect of these new therapies is shown in the table above.
The most common gastrointestinal symptom of these therapies, often called targeted therapies, is diarrhea, as shown in a study of cetuximab and irinotecan, where diarrhea occurred in approx. 50% of all patients.33 The mechanism seems unclear. Targeted therapy-induced diarrhea is responsible for dose-limiting toxicity (DLT).34
Some targeted therapies alter muscle mass due to interference with muscle metabolism pathways, for example, mammalian target of rapamycin (m-Tor) inhibitors are associated with sarcopenia.35 There is also some evidence that sarcopenia increases the DLT of sorafenib in renal cancer patients.36,37
Biotherapies cause different adverse reactions and toxicities than classical chemotherapy. These include sarcopenia and gastrointestinal disorders like diarrhea.
References
1 American Cancer Society. How Surgery Is Used for Cancer 2016 Available from: https://www.cancer.org/treatment/treatments-and-side-effects/treat-ment-types/surgery/how-surgery-is-used-for-cancer.html.
2 Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr. 2017;36(3):623-50.
3 Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Maeda S, Haraguchi N, et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann Surg Oncol. 2015;22 Suppl 3:S778-85.
4 Zheng HL, Lu J, Li P, Xie JW, Wang JB, Lin JX, et al. Effects of Preoperative Malnutrition on Short- and Long-Term Outcomes of Patients with Gastric Cancer: Can We Do Better? Ann Surg Oncol. 2017;24(11):3376-85.
5 American Cancer Society. How Is Chemotherapy Used to Treat Cancer? Available from: https://www.cancer.org/treatment/treatments-and-side-effects/treat-ment-types/chemotherapy/how-is-chemotherapy-used-to-treat-cancer.html. PDF downloaded on 30 July 2020.
6 American Cancer Society. How Chemotherapy Drugs Work. Available from: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemo-therapy/how-chemotherapy-drugs-work.html. PDF downloaded on 30 July 2020.
7 Maasberg S, Knappe-Drzikova B, Vonderbeck D, Jann H, Weylandt KH, Grieser C, et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology. 2017;104(1):11-25.
8 Attar A, Malka D, Sabate JM, Bonnetain F, Lecomte T, Aparicio T, et al. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: an AGEO prospective cross-sectional multicenter study. Nutrition and cancer. 2012;64(4):535-42.
9 Davidson W, Teleni L, Muller J, Ferguson M, McCarthy AL, Vick J, et al. Malnutrition and chemotherapy-induced nausea and vomiting: implications for practice. Oncology nursing forum. 2012;39(4):E340-5.
10 Malihi Z, Kandiah M, Chan YM, Hosseinzadeh M, Sohanaki Azad M, Zarif Yeganeh M. Nutritional status and quality of life in patients with acute leukaemia prior to and after induction chemotherapy in three hospitals in Tehran, Iran: a prospective study. J Hum Nutr Diet. 2013;26 Suppl 1:123-131.
11 Sanchez-Lara K, Ugalde-Morales E, Motola-Kuba D, Green D. Gastrointestinal symptoms and weight loss in cancer patients receiving chemotherapy. Br J Nutr. 2013;109(5):894-7.
12 Marinho EDC, Custodio IDD, Ferreira IB, Crispim CA, Paiva CE, Maia YCP. Impact of chemotherapy on perceptions related to food intake in women with breast cancer: A prospective study. PloS one. 2017;12(11):e0187573.
13 Aaldriks AA, van der Geest LG, Giltay EJ, le Cessie S, Portielje JE, Tanis BC, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol. 2013;4(3):218-26.
14 Ihara K, Yamaguchi S, Shida Y, Ogata H, Domeki Y, Okamoto K, et al. Poor nutritional status before and during chemotherapy leads to worse prognosis in unresectable advanced or recurrent colorectal cancer. Int Surg. 2015.
15 Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188-98.
16 Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920-6.
17 Wendrich AW, Swartz JE, Bril SI, Wegner I, de Graeff A, Smid EJ, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral oncology. 2017;71:26-33.
18 Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer.Eur Radiol. 2014;24(5):998-1005.
19 American Cancer Society. Radiation Therapy. Available from: https://www.cancer.org/treatment/treatments-and-side-ef-fects/treatment-types/radiation.html. PDF downloaded on 30 July 2020.
20 Gellrich NC, Handschel J, Holtmann H, Kruskemper G. Oral cancer malnutrition impacts weight and quality of life. Nutrients. 2015;7(4):2145-60.
21 Unsal D, Mentes B, Akmansu M, Uner A, Oguz M, Pak Y. Evaluation of nutritional status in cancer patients receiving radiotherapy: a prospective study. Am J Clin Oncol. 2006;29(2):183-8.
22 Langius JA, Bakker S, Rietveld DH, Kruizenga HM, Langendijk JA, Weijs PJ, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer. 2013;109(5):1093-9.
23 Webb GJ, Brooke R, De Silva AN. Chronic radiation enteritis and malnutrition. J Dig Dis. 2013;14(7):350-7.
24 Chaveli-Lopez B, Bagan-Sebastian JV. Treatment of oral mucositis due to chemotherapy. J Clin Exp Dent. 2016;8(2):e201-e209.
25 Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 Suppl):1995-2025.
26 Brown CG, Wingard J. Clinical consequences of oral mucositis. Semin Oncol Nurs. 2004;20(1):16-21.
27 Keefe DM, Rassias G, O'Neil L, Gibson RJ. Severe mucositis: how can nutrition help? Curr Opin Clin Nutr Metab Care. 2007;10(5):627-31.
28 Rosenthal DI, Mendoza TR, Fuller CD, Hutcheson KA, Wang XS, Hanna EY, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Cancer. 2014;120(13):1975-84.
29 Majdaeen M, Kazemian A, Babaei M, Haddad P, Hashemi FA. Concomitant boost chemoradiotherapy in locally advanced head and neck cancer: treatment tolerance and acute adverse reactions. J Cancer Res Ther. 2015;11(1):24-8.
30 Yamano T, Yoshimura M, Kobayashi M, Beppu N, Hamanaka M, Babaya A, et al. Malnutrition in rectal cancer patients receiving preoperative chemoradiotherapy is common and associated with treatment tolerability and anastomotic leakage. Int J Colorectal Dis. 2016;31(4):877-84.
31 American Cancer Society. Immune checkpoint inhibitors to treat cancer. Available from: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/immune-checkpoint-inhibitors.html. PDF downloaded on 30 July 2020.
32 Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin. 2013;63(4):249-79.
33 Vincenzi B, Santini D, Rabitti C, Coppola R, Beomonte Zobel B, Trodella L, et al. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: a single centre phase II trial. Br J Cancer. 2006;94(6):792-7.
34 Widakowich C, de Castro G, Jr., de Azambuja E, Dinh P, Awada A. Review: adverse reactions of approved molecular targeted therapies in solid cancers. The oncologist. 2007;12(12):1443-55.
35 Gyawali B, Shimokata T, Honda K, Kondoh C, Hayashi N, Yoshino Y, et al. Muscle wasting associated with the long-term use of mTOR inhibitors. Mol Clin Oncol. 2016;5(5):641-6.
36 Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21(8):1594-8.
37 Mir O, Coriat R, Blanchet B, Durand JP, Boudou-Rouquette P, Michels J, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PloS one. 2012;7(5):e37563.
38 Grant B. Nutritional Effects of Cancer Treatment: Chemotherapy, Biotherapy, Hormone Therapy and Radiation Therapy. In: Oncology Nutrition Dietetic Practice Group, Leser M, Ledesma N, Bergerson S, Trujillo E, editors. Oncology Nutrition for Clinical Practice. 2013:97-114.