Body weight
Body weight is the sum of muscle mass, fat mass, water and bone mass. Changes in body weight do not provide any information about the changes in these different components. Less food intake or higher requirement (negative nitrogen balance) in acute or chronic illness causes the body to use endogenous sources as fuel for metabolic reactions. Consequently, body weight declines.1
Measuring the amount of muscle mass is seen as elemental for assessing malnutrition, cachexia and sarcopenia.2,3,4 For example, sarcopenia in cancer patients can severely affect quality of life as well as negatively impact physical function and treatment tolerance.5
The assessment of muscle mass and fat reserves is preferably based on specific measurement methods (e.g., anthropometry, body impedance analysis (BIA) or imaging techniques like computed tomography (CT) scanning and dual energy X-ray absorptiometry (DEXA).5
3 different measurements for assessing body composition
It is assumed that these three play the most important role in the clinical practice of nutritional assessments. Cost, availability and ease of use can determine whether the techniques are better suited to clinical practice or are more useful for research.6
All measures include the following components:
Fat mass: Body fat content is the most variable component of the body, differing among individuals of the same sex, height and weight. On average, the fat content of women represents 26,9% of their total body weight compared with 14,7% for men.1
Fat-free mass (FFM): This is a mixture of water, minerals and protein, with the most protein stored in the muscle. Assessment of FFM is an indicator of muscle mass and can therefore provide an index of protein reserves in the body.1
Anthropometric measures
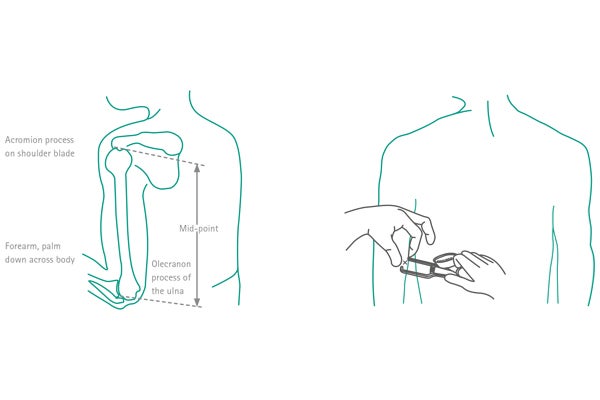
Most anthropometric methods used to assess body composition are based on a model in which the body consists of two chemically distinct compartments: fat mass and FFM.1
In population studies, body fat is often assessed by anthropometry. This measurement provides an estimation of the amount of subcutaneous fat stores, which in turn provides an estimation of total body fat. Fat mass is most commonly determined by anthropometry through measuring the thickness of the triceps, biceps, subscapular, suprailiac and midaxillary skin folds. Skin-fold thickness measurements are best made using precision skin fold thickness calipers.1,7
The measurement of the triceps skin fold is performed at the midpoint of the upper dominant arm, between the acromion of the upper right arm (between the acromion process and the tip of the olecranon) with the arm hanging relaxed1 (see Fig. 1).
Assessment of muscle mass
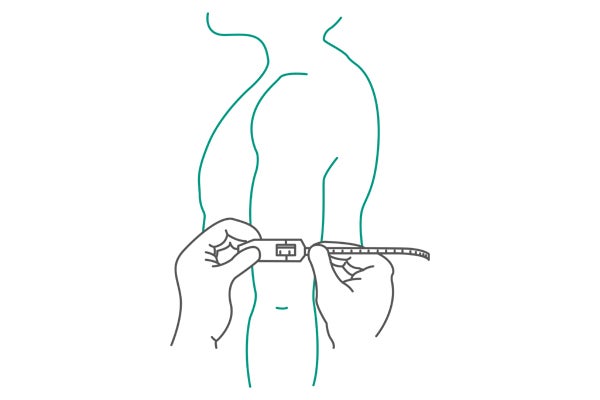
Mid-upper arm muscle circumference (MUAC) and mid-upper arm muscle area (MUAMA) correlate with the measures of total muscle mass. Therefore, equations are used to predict changes in protein nutritional status.1
The arm contains both subcutaneous fat and muscle. Arm circumference is easy to measure and requires a minimal amount of time and equipment. MUAC has been used for screening protein-energy malnutrition in emergencies such as famines and refugee crises. Measurements can be done with a flexible, nonelastic fiberglass tape measure1 (see Fig. 2).
To quantify the muscle mass with anthropometric measurements, the mid-upper arm circumference (MUAC) and the triceps skin fold (TSF) thickness are needed. Muscle mass can then be estimated using the corresponding formulas.1
The European Working Group on Sarcopenia in Older People (EWGSOP2) does not recommend the use of anthropometric measures for routine use in the diagnosis of sarcopenia, as they are prone to error.3,6
Advantages and limitations of anthropometry
| Advantages1 | Major limitations8 |
| simple handling | fluid shifts |
| cheap | changes in hydration status |
| non-invasive | interobserver variability |
Anthropometric measurements are simple but show a high variability and are not recommended for assessing muscle mass for sarcopenia.
BIA (bioelectrical impedance analysis)
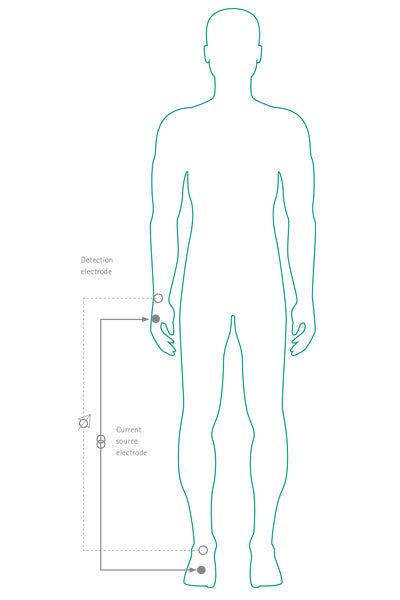
BIA is a measurement of body composition and depends on the difference in electrical conductance of FFM and fat mass. The technique measures impedance to an electrical current (typically 800 μA; 50 KHz). It uses resistance and reactance to estimate FFM and fat mass8,9 (see Fig. 3).
BIA is widely used in many clinical situations.9 The BIA-derived phase angle α (PhA) is determined by the relation between various measurements of resistance and reactance.9,10 PhA is interpreted as the membrane integrity indicator and a predictor of body cell mass. PhA has been studied as a prognostic tool in cancer patients.10 The EWGSOP2 does recommend BIA as one tool to quantify muscle quantity and to confirm the diagnosis of sarcopenia.3
Advantages and limitations of BIA
| Advantages9 | Major limitations8 |
| simple procedure | hydration status |
| portable equipment | electrolyte abnormalities |
| non-invasive | lack of standards for specific body sizes |
| the results are reproducible and rapidly obtained |
The precision can also be affected by recent physical activity, consumption of food or beverages, ambient air and skin temperature, menstrual status and body position1. High variability between patients which requires careful interpretation of results based on the individual patient.10
BIA is a validated and non-invasive tool to predict muscle mass. The BIA-derived phase angle is a prognostic factor. However, the values of BIA can be affected by many factors.
CT (Computerized tomography) scanning
Computerized tomography (CT) is based on the relationship between the degree of attenuation of an X-ray beam and the density of the tissues through which the beam has passed.1
The third lumbar vertebra (L3) is used as the standard landmark. The L3 region contains psoas, paraspinal muscles and the abdominal wall muscles. The structure of those specific muscles is quantified based on pre-established thresholds of Hounsfield units (-29 to +150) of skeletal muscle tissue.11
Cross-sectional areas (cm2) of the sum of all of these muscles are computed for each image. The directly determined unit is the area (cm2) of total L3 skeletal muscle which is linearly related to whole body muscle mass, and calculated as the L3 skeletal muscle index, in cm2 / m2.11
Sarcopenia predicted by CT scans in cancer patients has prognostic value.12 The European Working Group on Sarcopenia in Older People 2 (EWGSOP2) stated in 2010 that CT scanning technology is one of the gold standards to effectively measure muscle mass only for research.6 But a lot of research has been done in this field12-15 after 2010 so it may be expected that in the future CT scans used routinely for cancer staging could be used to predict sarcopenia.
Avantages and limitations of CT measurement
| Advantages3,11 | Major limitations3 |
| Skeletal muscle area (cm2) can be measured by using routine CT scans conducted for diagnostic purposes | high cost |
| limited access to equipment at some sites | |
| concerns about radiation exposure limit the use for routine clinical practice |
CT scans at the third lumbar vertebra used for routine purposes like diagnosing and staging can predict sarcopenia in cancer patients.
Nutritional assessments
References
1 Gibson RS. Principles of Nutritional Assessment. 2 nd ed: OXFORD University press; 2005.
2 Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49-64.
3 Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age ageing. 2019;48(1):16-31.
4 Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489-95.
5 Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11-48.
6 Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age ageing. 2010;39(4):412-23.
7 CDC. National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual 2007 [Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf. Download on 10. September 2020.
8 Pesce-Hammond K, Wessel J. Nutrition Assessment and Decision Making. In: Merritt R, editor. The ASPEN Nutrition Support Practice Manual. 2nd ed: American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.); 2005:3-37.
9 Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gomez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23(5):1226-43.
10 Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients – a comprehensive review. Eur J Clin Nutr. 2015;69(12):1290-7.
11 Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269-75.
12 Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58-67.
13 Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, et al. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin Cancer Res. 2017;23(3):658-65.
14 Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107(6):931-6.
15 Grossberg AJ, Chamchod S, Fuller CD, Mohamed AS, Heukelom J, Eichelberger H, et al. Association of Body Composition With Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA oncology. 2016;2(6):782-9.



